PNNL-developed solvent breaks barriers, captures carbon for less than industrial counterparts.As part of a marathon research effort to lower the cost of carbon capture, chemists have now demonstrated a method to seize carbon dioxide (CO2) that reduces costs by 19 percent compared to current commercial technology. The new technology requires 17 percent less energy to accomplish the same task as its commercial counterparts, surpassing barriers that have kept other forms of carbon capture from widespread industrial use. And it can be easily applied in existing capture systems.
In a study published in the March 2021 edition of International Journal of Greenhouse Gas Control, researchers from the U.S. Department of Energy’s Pacific Northwest National Laboratory—along with collaborators from Fluor Corp. and the Electric Power Research Institute—describe properties of the solvent, known as EEMPA, that allow it to sidestep the energetically expensive demands incurred by traditional solvents.
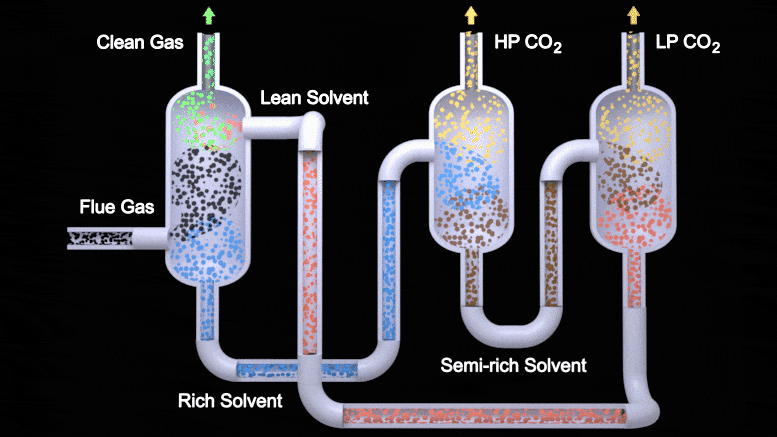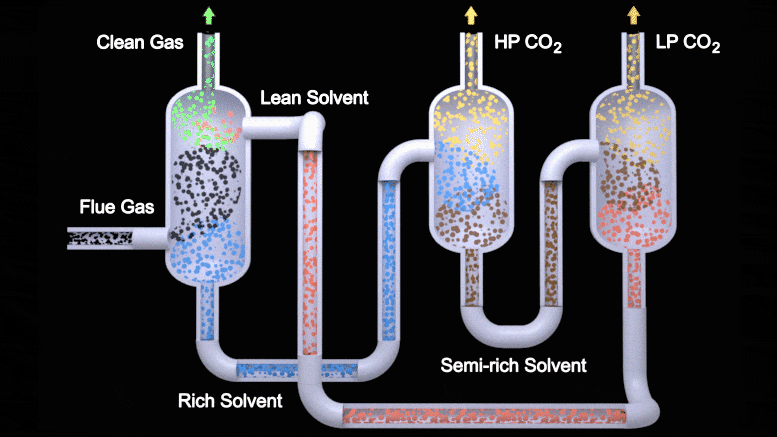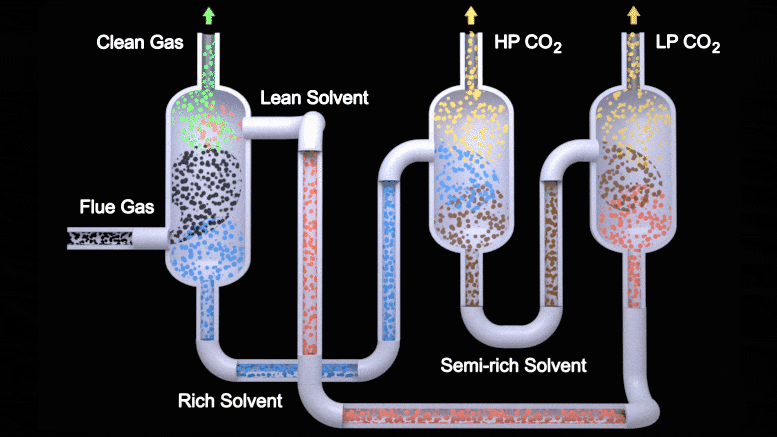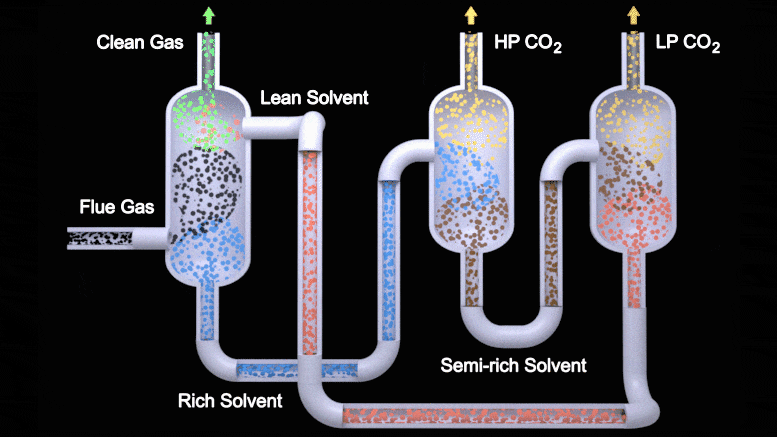
This animation depicts the two-stage flash configuration, one of several processes described in a new study detailing how EEMPA, a Pacific Northwest National Laboratory-developed solvent, can capture carbon from flue gas emitted by power plants. From left to right, EEMPA (red) first interacts with flue gas (black), where it absorbs carbon dioxide. Then, as a saturated solvent (blue), EEMPA is stripped of carbon dioxide in high and low-pressure tanks. Finally, the stripped solvent is reintroduced to the carbon dioxide absorber, where the process begins again. Credit: Animation by Michael Perkins | Pacific Northwest National Laboratory

